Speed up recruiting and ensure compliance with RealTime's eCONSENT
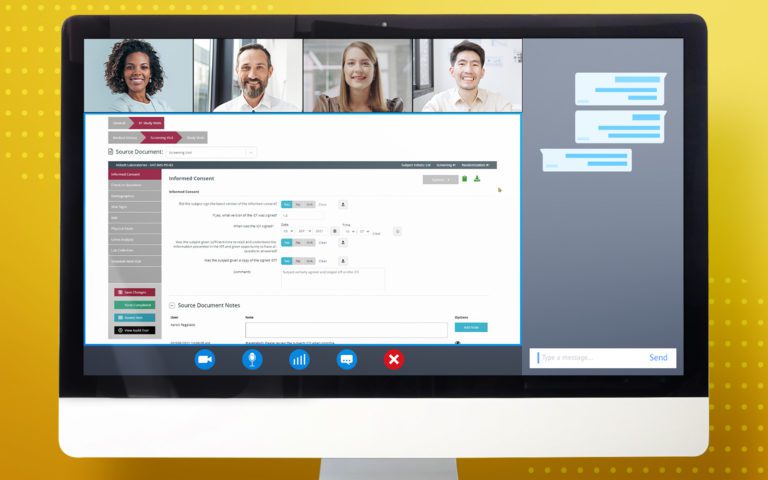
Enroll participants faster and more easily with remote consenting in RealTime’s 21 CFR Part 11 compliant Participant Portal that subjects can conveniently access via desktop or mobile app. The Participant Portal, MyStudyManager™, allows participants to enroll in a study, manage their participation and better engage with site staff in both traditional and Decentralized Clinical Trial (DCT) studies.
Alerts & Notifications
Eliminate missing consent deviations, one of the FDA's most common 483 findings.
Consent Form Routing
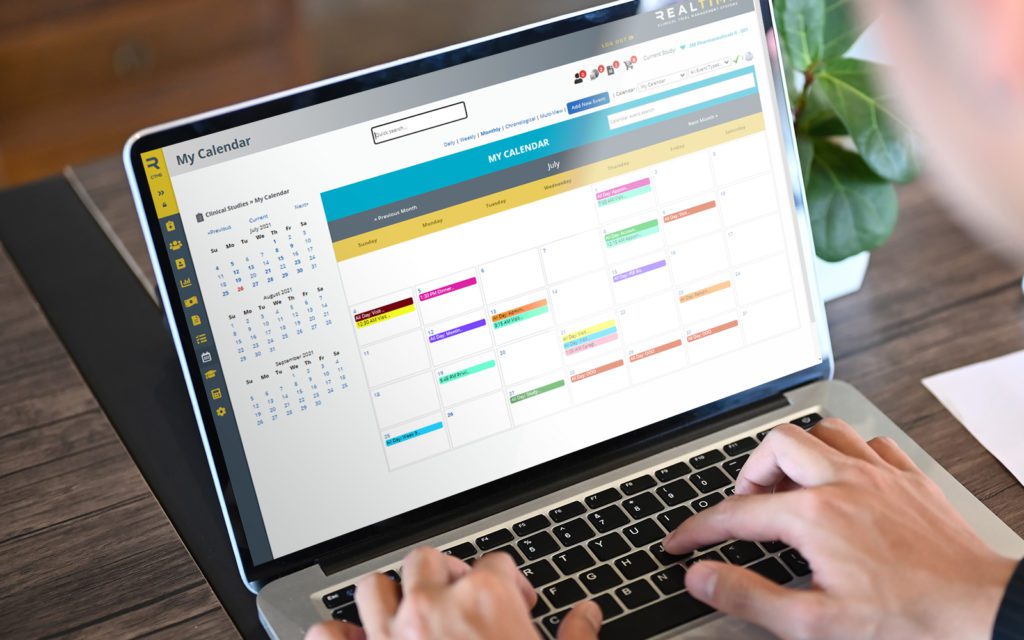
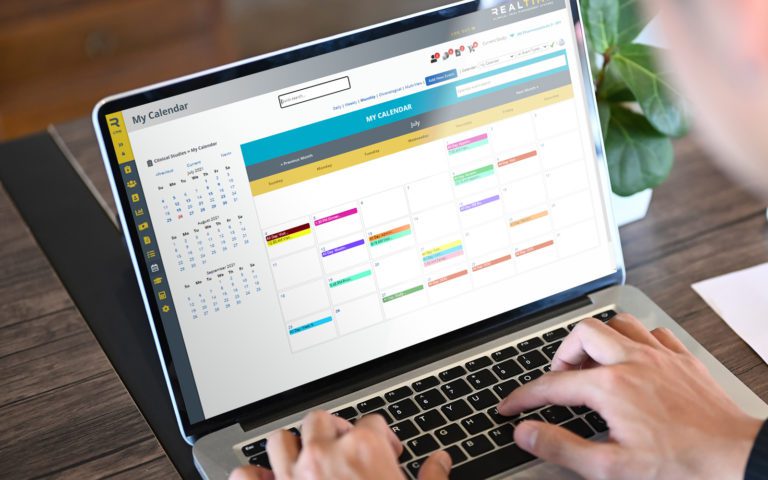
Save time with automated eCONSENT routing based on language, version and more. Plus, version control ensures that your staff is always signing the most current document.

Organize Signature Elements
Manage the order of signees and customize the placement of elements such as signature, date & time, and check boxes. Brought to you by RealTime's 'Sign on the Line' technology.
Maintain Compliance
Ensure that study staff is trained and delegated to perform consent responsibilities.


REMOTE eCONSENTING
Allow participants to consent anytime, anywhere to speed up study enrollment and support Decentralized Clinical Trials (DCT).
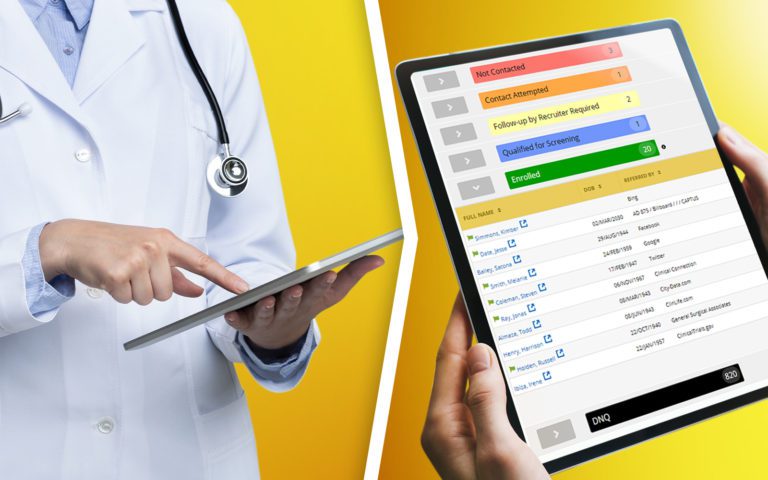
Consent Oversight
Quickly review the status of pending or completed signatures on each individual consent form.